Heavy Hanger Conveyor Belt,Metal Hanger Conveyor Belt,Conveyor Hanger,Hanger Conveyor Systems Changxing Huarui Machinery Equipmnet Co., Ltd. , https://www.huaruicoatingline.com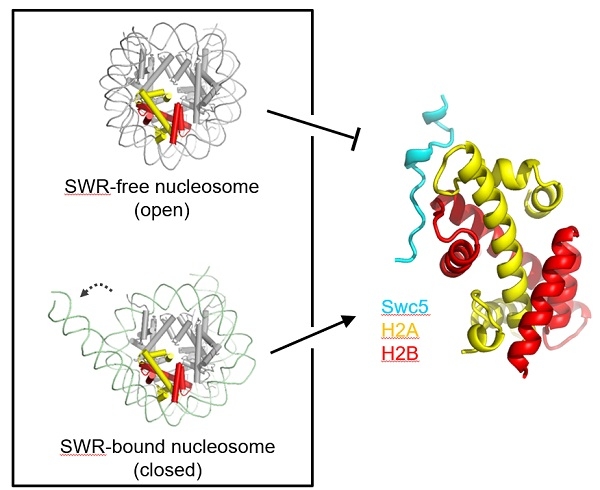
Institute of Biophysics Reveals New Mechanism of H2A.Z Chromatin Assembly
[ Instrument Network Instrument R & D ] On January 30, the Proceedings of the National Academy of Sciences (PNAS) published a research paper "Role of a DEF / Y motif in histone H2A-H2B" recognition and nucleosome editing ". This study revealed the molecular mechanism by which the SWR complex subunit Swc5 specifically recognizes histone H2A-H2B and regulates histone H2A.Z for chromatin assembly.
H2A.Z is a variant of histone H2A. H2A.Z in yeast and mammalian cells has highly conserved sequences and plays an important role in gene transcription, DNA replication, and maintenance of genome stability. H2A.Z changes the structure of chromatin and achieves its function by pinpointing specific locations in the genome. The chromatin remodeling complex SWR releases energy by hydrolyzing ATP, and gradually replaces H2A nucleosomes near the transcription start site with H2A.Z nucleosomes, thereby achieving chromatin localization of H2A.Z. In order to clarify the function of SWR and the chromatin localization mechanism of H2A.Z, Zhou Zheng's research group has conducted in-depth research on H2A.Z exchange reaction in recent years, and has revealed H2A.Z removal (Cell Res 2014), SWR subunit YL1 Regulation of H2A.Z assembly (Nat Struct Mol Biol 2016), histone chaperone Chz1 regulates H2A.Z assembly (PLoS Biol 2019) and other important mechanisms. Existing research shows that while SWR catalyzes the assembly of H2A.Z-H2B into nucleosomes, H2A-H2B will be removed from nucleosomes. In this process, the SWR subunit Swc5 plays an important role in the recognition of H2A-H2B, but its molecular mechanism is unclear.
By measuring the crystal structure of yeast-derived Swc5 and H2A-H2B, the researchers found that both Swc5 and its mammalian homologous protein CFDP1 use a tandem DEF / Y motif to recognize H2A-H2B. The structure shows that Swc5 uses a polar amino acid in the center and a hydrophobic amino acid at both ends to form its unique "tridentate mode" that firmly binds H2A-H2B, while ensuring that it selectively recognizes conventional histone H2A instead of variant histone H2A .Z. Through isothermal calorimetric titration, in vitro enzyme activity measurement, and in vivo cross-linking experiments, the researchers confirmed that the DEF / Y motif plays an important role in identifying H2A-H2B, promoting histone exchange, and regulating the catalytic activity of SWR. This study suggests that when the SWR complex catalyzes the H2A.Z exchange reaction, the nucleosome DNA opens and exposes the Swc5 binding site on H2A-H2B. The recognition and binding of Swc5 to H2A-H2B will promote the latter from the nucleosome. Removed to ensure the smooth progress of the H2A.Z exchange reaction (Figure 1). This study laid the foundation for elucidating the function of the SWR complex and the chromatin localization mechanism of H2A.Z.
Zhou Zheng, a researcher at the Institute of Biophysics, and Ed Luk, a professor at Stony Brook University in the United States, are co-corresponding authors. Huang Yan, PhD student of Zhou Zheng's group, is the first author of the thesis. Lu Sun, Leonidas Pierrakeas of Ed Luk's group, Dai Linchang, special research assistant of Zhou Zheng's group, and Pan Lu, assistant researcher also participated in the study. This research was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, and the Strategic Pilot Technology Special Project (Class B) of the Chinese Academy of Sciences. The Shanghai Synchrotron Radiation Light Source (SSRF) and the experimental platform of the Institute of Biophysics provided important technical support for the research.